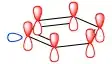
If benzene were to donate a hydrogen ion, then could the negative charge still be delocalised around the ring? Wouldn't that break aromaticity and thus destabilise the molecule?
Asked
Active
Viewed 3,152 times
1 Answers
3
See: Relative acidities of alkanes, alkenes, and alkynes. Essentially the same explanation holds here, as benzene carbons are sp2-hybridised.
The newly formed lone pair is in a σ-type orbital (blue) which does not overlap with the π system (red), hence no delocalisation is possible.
orthocresol
- 71,033
- 11
- 239
- 410