The compound 2,3,7,8-tetramethylspiro[4.4]nonane has five potential stereogenic or pseudoasymmetric centres: the four C atoms with the methyl substituents and the spiro atom. In total there are $2^5=32$ possible configurations. However, many of these combinations are actually identical compounds.
A simple and obviously exhaustive (but also exhausting) approach is to draw all 32 configurations and name them. (This approach may also be used to check the results if someone finds a more elegant solution.) The resulting names for the 32 combinations are:
- (2R,3R,7R,8R)-2,3,7,8-tetramethylspiro[4.4]nonane (2 times)
![(2R,3R,7R,8R)-2,3,7,8-tetramethylspiro[4.4]nonane](../../images/91cb4b8078faabe4cce964d20fd23081.webp)
- (2R,3R,7R,8S)-2,3,7,8-tetramethylspiro[4.4]nonane (8 times)
![(2R,3R,7R,8S)-2,3,7,8-tetramethylspiro[4.4]nonane](../../images/5119cb0d20ae3325985ed53b93627c5f.webp)
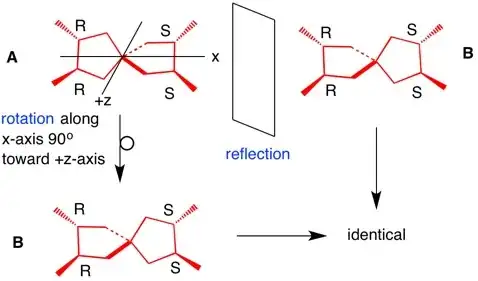
- (2R,3R,7S,8S)-2,3,7,8-tetramethylspiro[4.4]nonane (4 times)
![(2R,3R,7S,8S)-2,3,7,8-tetramethylspiro[4.4]nonane](../../images/d36d7bb4670e24cdf37b25f48a821b40.webp)
- (2R,3S,5r,7R,8S)-2,3,7,8-tetramethylspiro[4.4]nonane (4 times)
![(2R,3S,5r,7R,8S)-2,3,7,8-tetramethylspiro[4.4]nonane](../../images/1dfd3d3190ee4cc668ccdcf8c537d10c.webp)
- (2R,3S,5s,7R,8S)-2,3,7,8-tetramethylspiro[4.4]nonane (4 times)
![(2R,3S,5s,7R,8S)-2,3,7,8-tetramethylspiro[4.4]nonane](../../images/bedda68c8229a06cfab5253cb055522a.webp)
- (2R,3S,7S,8S)-2,3,7,8-tetramethylspiro[4.4]nonane (8 times)
![(2R,3S,7S,8S)-2,3,7,8-tetramethylspiro[4.4]nonane](../../images/d38a40977044c1a970fd75db4cb41632.webp)
- (2S,3S,7S,8S)-2,3,7,8-tetramethylspiro[4.4]nonane (2 times)
![(2S,3S,7S,8S)-2,3,7,8-tetramethylspiro[4.4]nonane](../../images/c1dbfc925304c58047edbc9d301722f2.webp)
Therefore, there are seven different combinations.
![2,3,7,8-tetramethylspiro[4.4]nonane](../../images/0ca36d96a0ba48c571a5e66b1e60a0e2.webp)
![(2R,3R,7R,8R)-2,3,7,8-tetramethylspiro[4.4]nonane](../../images/91cb4b8078faabe4cce964d20fd23081.webp)
![(2R,3R,7R,8S)-2,3,7,8-tetramethylspiro[4.4]nonane](../../images/5119cb0d20ae3325985ed53b93627c5f.webp)
![(2R,3R,7S,8S)-2,3,7,8-tetramethylspiro[4.4]nonane](../../images/d36d7bb4670e24cdf37b25f48a821b40.webp)
![(2R,3S,5r,7R,8S)-2,3,7,8-tetramethylspiro[4.4]nonane](../../images/1dfd3d3190ee4cc668ccdcf8c537d10c.webp)
![(2R,3S,5s,7R,8S)-2,3,7,8-tetramethylspiro[4.4]nonane](../../images/bedda68c8229a06cfab5253cb055522a.webp)
![(2R,3S,7S,8S)-2,3,7,8-tetramethylspiro[4.4]nonane](../../images/d38a40977044c1a970fd75db4cb41632.webp)
![(2S,3S,7S,8S)-2,3,7,8-tetramethylspiro[4.4]nonane](../../images/c1dbfc925304c58047edbc9d301722f2.webp)