The idea of a 'rotatable bond' is somewhat arbitrary, given that under the right conditions basically any bond can be rotated. When thinking about concepts in medicinal chemistry like rotatable bonds, its therefore important to make sure you're working with the definition used when defining the rule.
The original Veber papers (J. Med. Chem. 2002, 45, 2615) define what they consider to be a rotatable bond:
Rotatable bonds were defined as any single bond, not in a ring, bound to a nonterminal heavy (i.e., non-hydrogen) atom. Excluded from the count were amide C-N bonds because of their high rotational energy barrier.
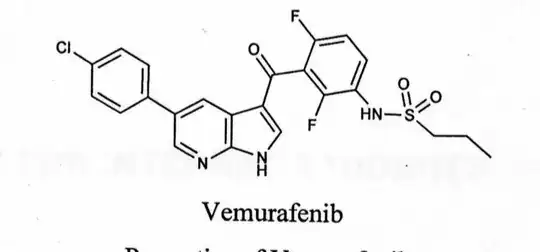
Based on this rule, the Ar-Ar single bond would count as a rotatable bond, as would the Ar-N bond. The sulfonamide is generally excluded for the same reason as amide C-N bonds (although I can't find a quote by Veber that actually discusses this).
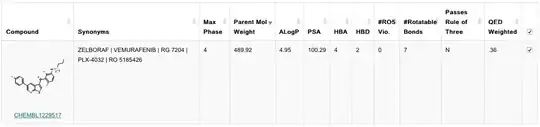
In reality, these properties are usually calculated automatically, for instance using ChEMBL.