I would like to give a very rough answer as to why equivalent resonance structures have a stabilizing effect.
To begin, we assume that resonance can be represented as a discrete n-state system. Feynman has used this simple approximation to explain many of the essential features of chemical bonding and resonance in the Feynman Lectures on Physics Vol III, Chapters 8 - 10.
To simplify we begin with a molecule which can have 2 different canonical structure based on the usual rules. Let the energies of the two states be $E_1$ and $E_2$ .
The Hamiltonian of this two state system will be:
$$H = \pmatrix{E_1& 0\\0&E_2}$$
The canonical structure are themselves eigen-states here. However in the real world we find more symmetry to the molecule/different bond lengths/angles than that is predicted by our canonical structures. This is because the two canonical structures are not the actual eigen states.
We assume that the new eigen states however can be represented as a superposition of the 'old' states.
Therefore in the most general case the Hamiltonian expressed in the 'old' base states become:
$$H = \pmatrix{E_1 & H_{12}\\H_{12}^*&E_2}$$
We find the energy eigenvalues from this matrix.
The energy eigenvalue with the lower energy in this case is:
$$
E^{'}_1 =
\frac{E_1 + E_2}{2}
- \frac{\sqrt{(E_1 - E_2)^2 + 4H_{12}H_{12}^*}}{2}
$$
Here without loss of generality let us assume that $E_2 \ge E_1$
So taking the difference $D = E_1 - E^{'}_1$ which is the energy difference between the old base state and the new eigenstate with lower energy:
$$
D =
E_1 - E^{'}_1 =
\frac{E_1-E_2}{2}
+ \frac{\sqrt{(E_1 - E_2)^2 + 4H_{12}H_{12}^*}}{2}
$$
This $D$ is a measure of the stabilization due to resonance.
Setting $E_1 - E_2 = x \le 0$, we get
$$D = \frac{x}{2} + \frac{\sqrt{x^2 + 4H_{12}H_{12}^*}}{2}.$$
The graph of this function is:
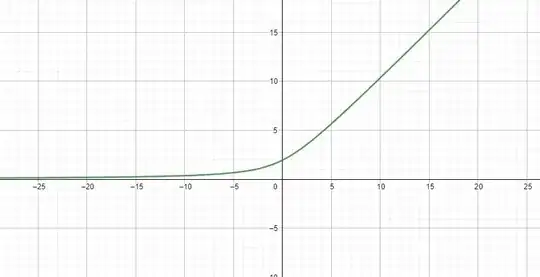
So we see that for $x \le 0$ , $D$ is maximum when $x = 0$, i.e. $E_1 = E_2$. One way this can happen is when our base states are symmetrical or in other words when the molecule has equivalent resonance structures.
However one should be careful of the comparison being made here. We are not comparing the molecule to some other molecule. All that is said here is that the stabilization of the molecule due to resonance is maximum when the canonical structures are equivalent (for a 2-state system).



