Tautomers are constitutional isomers of (usually organic) compounds that readily interconvert into each other. This tag is also suitable for tautomerization, the isomerization by which tautomers are interconverted. It may synonymously specified for questions about tautomerism.
Tautomers are constitutional isomers of (usually organic) compounds that readily interconvert into each other. The process of this interconversion is called tautomerization, which is a heterolytic molecular rearrangement. This process can quite frequently be very rapid (comp. goldbook).
The IUPAC goldbook describes tautomerism as an isomerism of the general form $$\ce{G-X-Y=Z <=> X=Y-Z-G},$$
where $\ce{X, Y, Z}$ are typically any of the $\ce{C, H, O, S}$ elements and $\ce{G}$ becomes electrofuge or nucleofuge. The most common case is, when the electrofuge is a proton, $\ce{H+}$, is also known as prototropy. Very common are the tautomerization of (iso-)cyanic acids:
\begin{align}
\ce{H-C#N &~<=>~ {}^{\ominus}C#N^{\oplus}-H} \tag{1}\\
\ce{O=C=N-H &~<=>~ N#C-O-H}\tag{2}
\end{align}
The case of $(1)$ it is also known as dyadic tautomerism, where the proton migrates between neighbouring atoms.
Other very well known cases are keto-enol and lactam-lactim tautomerism:
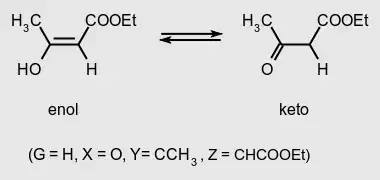
(graphics source: goldbook)