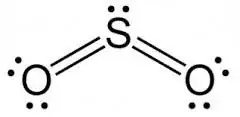
I recently came across this sentence in my textbook:
the bonds between sulphur and oxygen in oxides of sulphur ($\ce{SO2}$ and $\ce{SO3}$) are much shorter than might be expected for a single bond.
I feel it could be due to partial double bond character due to resonance.
And the same textbook gives another explanation:
In these molecules, in addition to normal π bond, a π bond is also formed by the sidewise overlap of a filled 2p orbital of oxygen with a vacant 3d orbital on the sulphur). This is called pπ - dπ bond and results in bringing the two atoms closer and thus accounts for shorter bond length of $\ce{S-O}$ bond.
The reason provided in the textbook (as mentioned above) is definitely untrue and incorrect
Because of reasons mentioned in these posts
Why is the bond order in the SO₃ molecule 1.33 and not 2? . Hybridization of sulfur in sulfur dioxide
Please provide a explaination for the same (the title question).