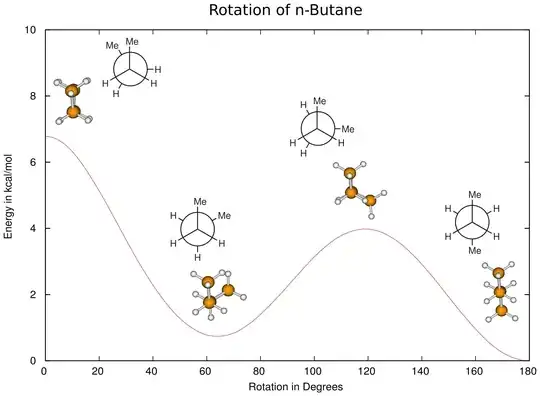
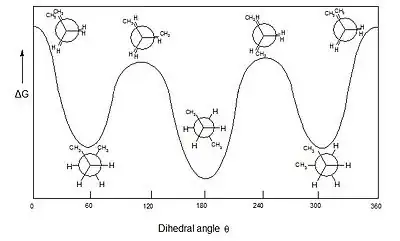
Would these be valid conformations of butane? I tried matching these up with the group but my drawings all seem rotated versus the book.
I'm fairly sure my anti conformer is correct, along with my gauche conformer. I'm not too sure about the two eclipsed conformers; from what I understand, there are two eclipsed conformations - one with strong torsional strain resulting from the methyl group interacting with the other methyl group, and the other resulting from the methyl groups interacting with the hydrogens, right?
Also, can we consider the dihedral angle to be the angle between the methyl groups?

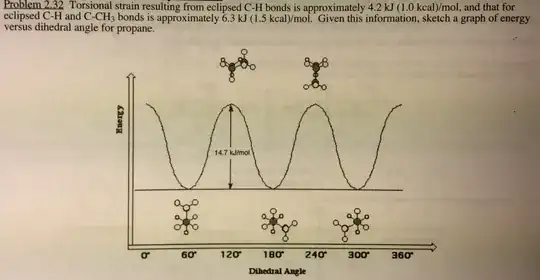
Another problem is that what I've drawn doesn't seem to match with the book's energy diagram too. I'd expect the anti-conformer to be the lowest in energy, but I'd also expect a difference in E for the two eclipsed conformations. One should have more torsional strain from placing the two methyl groups next to each other and the other, less, because the methyl groups are eclipsing the comparatively small hydrogen.