As already explained in a related answer, the most important simplified criteria for the numbering in such cases are:
- lower locants for the principal characteristic group that is expressed as suffix
- lower locants for multiple bonds
- lower locants for prefixes
- lower locants for substituents cited first as a prefix in the name
The corresponding actual wording in the current version of Nomenclature of Organic Chemistry – IUPAC Recommendations and Preferred Names 2013 (Blue Book) reads as follows:
P-14.4 NUMBERING
When several structural features appear in cyclic and acyclic compounds, low locants are assigned to them in the following decreasing order of seniority:
(…)
(c) principal characteristic groups and free valences (suffixes);
(…)
(e) saturation/unsaturation:
(i) low locants are given to hydro/dehydro prefixes (…) and ‘ene’ and ‘yne’ endings;
(ii) low locants are given first to multiple bonds as a set and then to double bonds (…);
(f) detachable alphabetized prefixes, all considered together in a series of increasing numerical order;
(g) lowest locants for the substituent cited first as a prefix in the name;
(…)
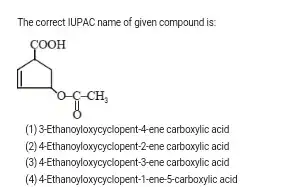
The principal characteristic group is the carboxylic acid. Therefore, the lowest locant is assigned to the $\ce{-COOH}$ group in accordance with Rule (c).
Next, a low locant is assgined to the double bond according to Rule (e). Therefore, the name of the structure without any further substituents is cyclopent-2-ene-1-carboxylic acid.
Finally, a low locant is assgined to the remaining substituent to Rule (f).
Thus, the correct name is 4-(acetyloxy)cyclopent-2-ene-1-carboxylic acid.