This is the structure of carvone, with the chiral carbon noted by *.
I don't understand why this is a chiral carbon, to my understanding a chiral carbon is a carbon with 4 different chemical groups attached to it.
In the molecule, the chiral carbon is surrounded by $\ce{H}$, $\ce{C(CH3)=CH2}$ and two $\ce{CH2}$ groups.
Why are both $\ce{CH2}$ groups different and thus making that carbon chiral?
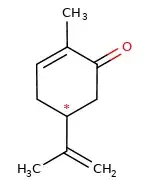
If that double bond in the cyclic part wasn't there that would mean that carbon 3 and 4 (counting anticlockwise from the ketone) would be the same, but because carbon 3 is closer to the electronegative element it counts as a different group in terms of chirality, is that right?
– Rafael Franco May 07 '19 at 14:45