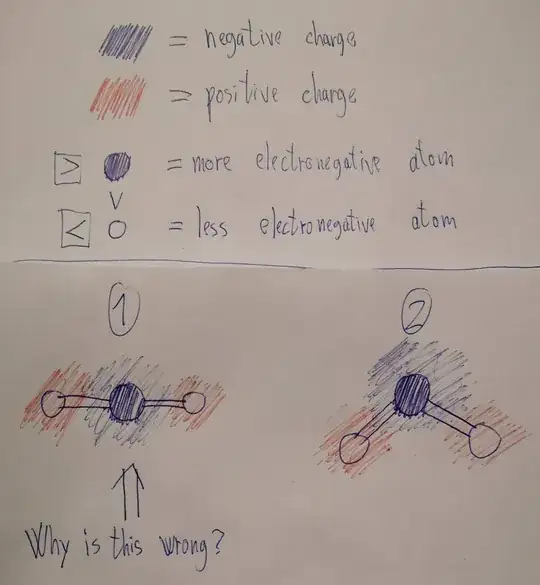
As I understand, polarity in molecules comes from the difference in electronegativity or the ability of the atoms to attract eletrons so the electrons spend more time or are more probable to gravitate more around the atom with stronger electronegativity right? So I wonder why polar molecules must necessarily have an asymetrical structure? (as I've read on multiple sites). Shouldn't a symetrical atom with strong electronegativity with 2 atoms bonded linearly 180° on both sides which are significantly less electronegative, be polar since the electrons would gravitate more towards the center and the sides would then be more positively charged? What am I missing here?
Why is the first molecule (1) not possible? Is it becuase of the less polar atom on the left side repells electrons just as much as the one on the right? But then the electrons would still orbit the middle atom more because its more elctronegative, right?