This is a specific example of the Silver Nitrate Test, one of the experiments used in organic chemistry teaching laboratories. Usually, the Silver Nitrate Test allows for the identification of alkyl halides by observing them in an alcoholic silver nitrate environment. For change, here used aqueous environment instead. In general, You will test the reactivity of several alkyl halides in a $\mathrm{S_N1}$ reaction. The silver ion (a halophilic reactant) complexes with the halide and precipitates out of solution first (note that silver nitrate in aqueous or alcoholic solution promotes ionization of the alkyl halide), and resulting carbocation react with ethanol to form ethyl ethers (in your case, it forms alcohol with water). The rate of the silver halide salt precipitation is characteristic of different types of alkyl halides ($3^\circ \gt 2^\circ \gt 1^\circ$).
When silver nitrate is used with $1^\circ$- or $2^\circ$-alkyl halides, a rearrangement may occur before the product formation stage. For example:
$$\ce{(CH3)3CCH2-Br + H2O + AgNO3 -> (CH3)2C(OH)CH2CH3 + AgBr + HNO3}$$
Rearrangements will only occur when the resulting carbocation is more stable than the initial carbocation. For example, see a relevant reaction below (Ref.1):

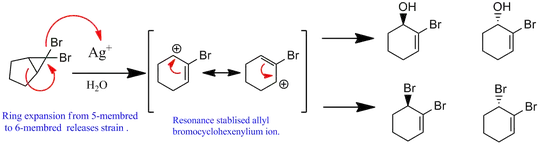
In your case, $\ce{Ag+}$ ion act as a Lewis acid and abstract one of bromide to leave bromocyclopropyl carbocation which is also comparatively stable cation (see the ring expansion in the diagram). The driving force here to rearrange the structure is releasing the strain energy, which gives you a relatively stable allyl cylohexenyl carbocation. It would then react with a water molecule to give the compound 2 as the final product. The same result was obtain from this reaction and has been reported (Ref.2), abstract of which states that:
Several ring expansion products carrying vinylic bromo functionality were synthesized by opening of the geminal dibromobicyclo[n.1.0]alkanes ring. Dibromocarbene was formed from bromoform and potassium tert-butoxide in hexane. Its reaction with various cyclic alkenes was the resultant dibromobicyclo[n.1.0]alkanes. Then, opening was performed using $\ce{AgNO3}$ in various solvent systems, such as acetic acid/DMSO, acetic acid/DMF, $\ce{CH3OH}$/acetone, and $\ce{H2O}$/DMF.
References:
- Russell J. Hewitta, Joanne E. Harvey, “Synthesis of C-furanosides from a D-glucal-derived cyclopropane through a ring-expansion/ring-contraction sequence,” Chem. Comm. 2011, 47(1), 421–423 (DOI: 10.1039/C0CC02244F).
- Mesut Boz, Hafize Çalişkan, Ömer Zaím, “Silver Ion-Assisted Ring Expansions in Different Solvent Systems,” Turk. J. Chem. 2009, 33(1), 73–78 (DOI: 10.3906/kim-0807-3).