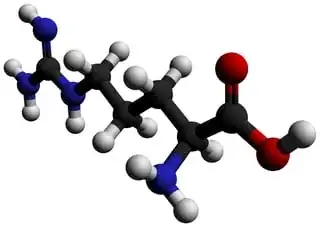
L-arginine is the most basic amino acid since it carries guanidyl group in addition to the $\alpha$-amino group. The arginine molecule is a zwitterion with the guanidyl group, rather than the primary $\alpha$-amino group, accepting extra proton from carboxylic acid group (Ref.1). Protonated or not, when consider guanidine as a molecule, there shouldn't be any $\mathrm{sp^3}$ nitrogen in the molecule because it is resonance-stabilized molecule. As a guanidyl group, this principle stays the same and it stays flat. There are few excellent answers to similar question you could find in here. In one answer it states that:
Guanidine is isolobal to urea, where the carbonyl oxygen has been replaced by an imine $\ce{NH}$. However, in principle it is still the same flat, resonance-stabilised molecule. The main difference is that there is no ‘preferred’ site for the double bond — it could point towards any of the three nitrogens in theory; you could say the diamide resonance is enhanced (Author: Jan).
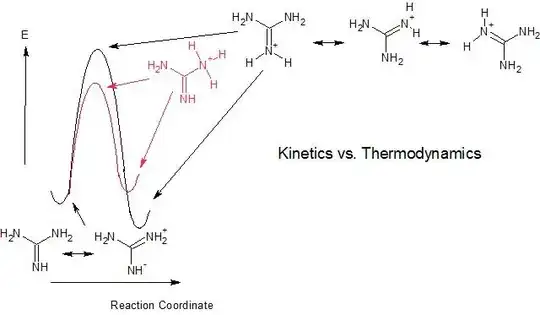
And, accepted answer showed following diagram to depict the concept of resonance stabilization (Author: Ron):
Further, I'll attached recent crystal structure of L-arginine, which shows clear equivalent three nitrogen atoms with hydrohen bonding network (Ref.2):

Two planes characterize the arginine molecule: one through the acid group and the other through the extended side chain, which contains the guanidyl group. The dihedral angle between these two planes is $74^\circ$ (Ref.1).
References:
- I. L. Karle, J. Karle, “An application of the symbolic addition method to the structure of L-arginine dehydrate,” Acta Cryst. 1964, 17, 835–841 (https://doi.org/10.1107/S0365110X64002250).
- Emilie Courvoisier, P. Andrew Williams, Gin K. Lim, Colan E. Hughes, Kenneth D. M. Harris, “The crystal structure of L-arginine,” Chem. Commun. 2012, 48, 2761–2763 (DOI: 10.1039/C2CC17203H).