When 4-methylcyclopent-1-ene reacts with bromine, then 1,2-dibromo-4-methylcyclopentane forms. And I am stuck on determining the amount of stereoisomers that can form as a result.
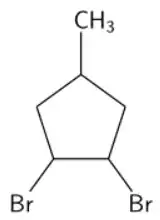
1,2-dibromo-4-methylcyclopentane has two chiral carbon atoms, those of bromine. And an internal mirror plane, we should have 3 optical isomers.
But I am confused upon drawing the following:

1 and 4 appear to be the same, 2 and 3 appear to be the same.
5 and 7 appear to be the same, 6 and 8 appear to be the same.
This gives me 4 isomers. So I draw them different again:

The puckered structure leaves me doubting myself: while I think that the internal mirror plane causes there to be only 2 different cis structures instead of 4 (or instead of 1 if it had been 1,2-dibromo-4,4-dimethylcyclopentane), I am not sure if the trans structures are actually the same.
