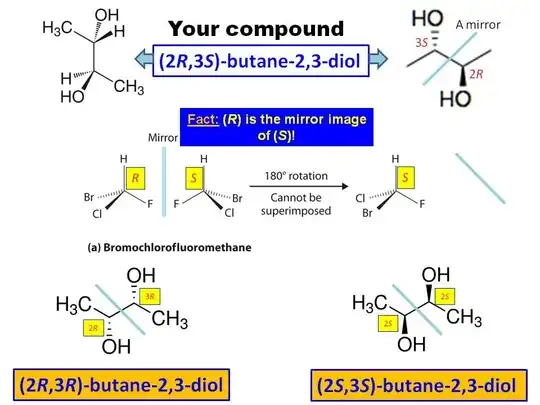
In a particular question in one of my reference book, I was asked if the given compound (meso-butane-2,3-diol) was a meso compound or not. In it’s solution part, to show that the compound was actually meso, they did the following.
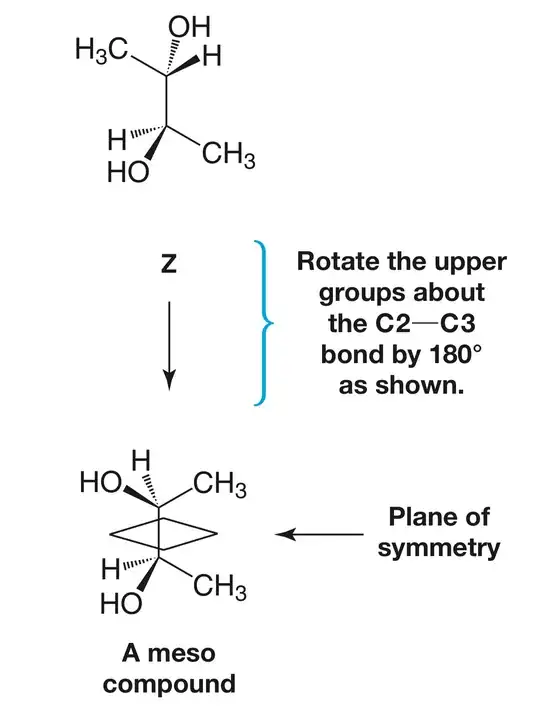
As I was not convinced about rotation of C2, I searched the web where I found this which says
While rotating the bonds about any atom (chiral) the stereochemistry of the molecule does not changes.
This is what I am confused. I mean if we rotate meso-butane-2,3-diol in the given way such that we have a conformer other than eclipsed or staggered, wouldn’t it be optically active, without any plane or centre of symmetry, which means rotation does change stereochemistry. Where am I going wrong?