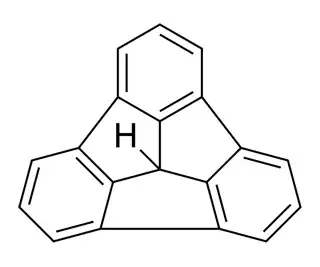
This is a question I've been thinking about quite frequently. I had seen that this hydrocarbon has a low $\mathrm pK_\mathrm a$ with reference to the highlighted H-atom
Loss of the central gives aromatic character to the three 5-membered rings in the compound, contributing to its acidity.
Are there any other neutral hydrocarbons which are stronger acids? How do their $\mathrm pK_\mathrm a$'s stack up against, say another organic acid such as acetic acid?
EDIT: As pointed out in the comments, $\ce{CH_5^+}$ (methanium) is a superacid, but is not a neutral hydrocarbon.