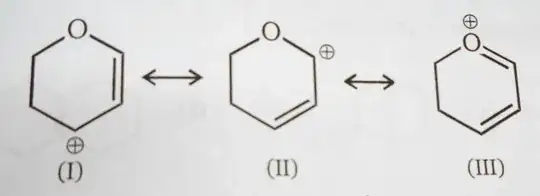
According to my book the answer is given that structure III is the most stable because all the atoms have complete octets.
But I think it should be II because of,
(a) Structure III has positive charge on a highly electronegative atom and
(b) In structure II, the positive charge can be stabilized by the Indicutive effect of Oxygen as well as resonance.
Can anyone provide a more convincing explanation as to why III is more stable?