Also, is it possible to see frame-shortening effects on electrostatic forces/van der waals at the edges, or are molecules too weak or necessarily neutral to be manipulated this way?
1 Answers
Rotation speed cannot be made arbitrarily close to the speed of light. Molecular rotation is quantized, and every molecule has a finite number of rotational levels, just like it has a finite number of vibrational levels based on the shape of its potential energy surface (the same can be said of vibrations: the molecule cannot vibrate arbitrarily quickly without dissociating).
However forces like the van der Waals force do get noticeably affected at the highest supported molecular energy levels. Specifically the $C_6/r^6$ van der Waals force becomes $C_7/r^7$ at certain distances, due to the Casimir-Polder effect in quantum electrodynamics (an intrinsically relativistic theory of the quantum world). The reason for this is that the derivation of the $C_6/r^6$ van der Waals force does not take into account the fact that information cannot travel at infinite speed, so it actually takes a finite amount of time (based on the finite speed of light) for one atom to "feel" the force of a distant atom. This "slowing down" of the energy transfer, is called "relativistic retardation" because "retardation" means slowing down. This causes the force to become weaker for larger distances ($1/r^7$ is much smaller than $1/r^6$ for larger $r$).
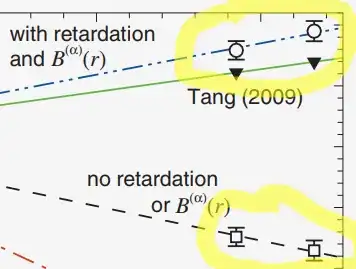
This is usually not taught at the undergraduate level, or even graduate level courses, so there is no easy "textbook" example to demonstrate it, so unfortunately we have to resort to publications in scientific journals, where the audience is far more advanced: But in the image below, I hope you can at least see that "with retardation" the experimental values here are much closer to the theoretical values of Tang (2009), versus the experimental values derived from a model "without retardation":
This shows evidence that the theoretical idea of "relativistic retardation" is in fact a much better description of the experiment than the non-relativistic theory, so it is indeed fair to say that the effect of light (electromagnetic radiation) having finite (rather than infinite) speed is observed in a molecule. The molecule here was Li$_2$ and the above image is from Figure 6 of this paper which deals with deriving information from experimental spectra of a molecule:
- 1,422
- 14
- 28
-
Comments are not for extended discussion; this conversation has been moved to chat. – Martin - マーチン Jan 26 '20 at 19:10