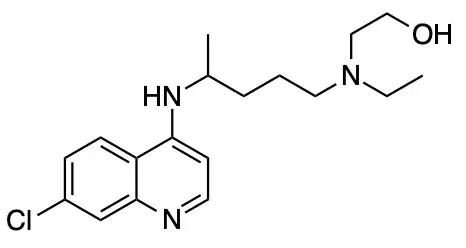
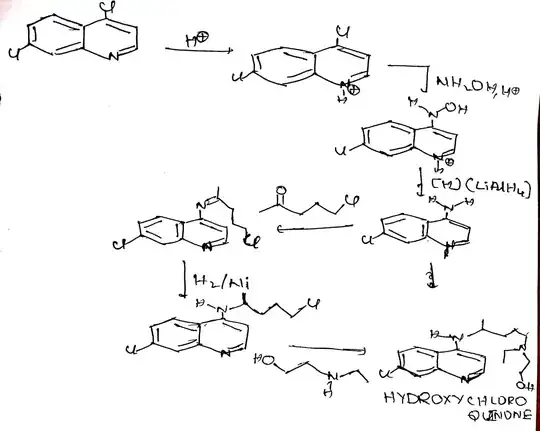
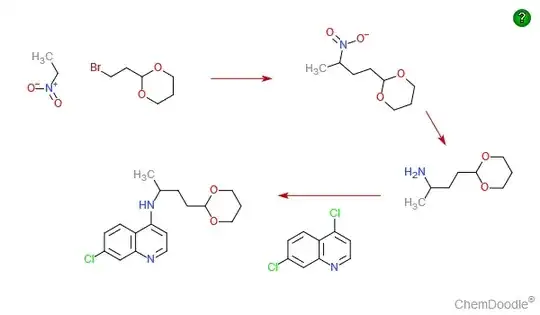
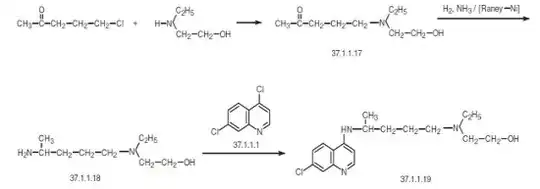
Note: Organic chemistry is not my field of expertise. This is just a supporting answer to Waylander's excellent answer which is purely based on online research. OP want some canonical answers and if people are interested in this question, I might as well place a bounty.
One route I found is react 1-chloro-4-pentanone with 2-ethylaminoethanol to make aminoketone which undergoes reductive amination making 4-[ethyl(2-hydroxyethyl)amino]-1-methylbutylamine. Reacting this with 4,7-dichlroquinoline* makes the desired hydroxychloroquine.
*There are 3 methods to make 4,7-dichloroquinoline(see ref. below for more details). One of the method is as follows:
Take 3-chloroaniline and ethoxymethylenmalonic ester to make (3-choroanilino)-methylenemalonic ester, which then undergoes high-temperature heterocyclization to make the ethyl ester of 7-chloro-4-hydroxyquinolin-3-carboxylic acid. Hydrolyzing this with sodium hydroxide gives 7-chloro-4-hydroxyquinolin-3-decarboxylic acid, which when heated at 250–270°C is decarboxylated, forming 7-chloro-4-hydroxyquinoline. Treating this with phosphorus oxychloride gives 4,7-dichloroquinoline.

Reference
- Hydroxychloroquine and Chloroquine articles respectively of ScienceDirect.com. Original reference is "Drugs for Treating Protozoan Infections by R.S. Vardanyan, V.J. Hruby, 2006"