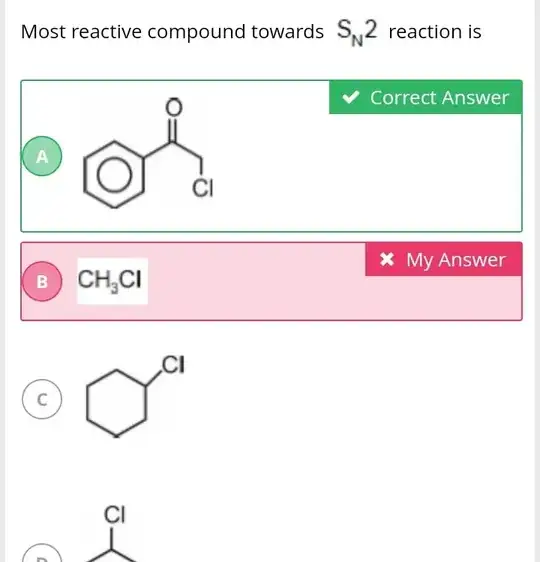
[!well i thought that sterically least hindered compound will undergo sn2 most easily but in this case it the compound having benzene ring is undergoing sn2 more easily although it will repel the attacking nucleophile due to its electron dense nature.why isnt that happening here??][1]][1]
Asked
Active
Viewed 44 times
0
-
@Anket Aulakh Check this link: https://chemistry.stackexchange.com/questions/48872/how-are-sn2-transition-states-stabilised-by-adjacent-double-bonds-and-carbonyl-g – Nikhil Anand Apr 27 '20 at 06:23
-
https://chemistry.stackexchange.com/questions/87827/why-are-alpha-carbonyl-halides-most-reactive-towards-sn2-reactions – Nikhil Anand Apr 27 '20 at 06:23