With regard to numbering of locants within the same compound class, multiple bonds have seniority over simple substituent groups (e.g. methyl, ethyl, etc.) that are used for naming of branched alkanes. On this matter, the current version of Nomenclature of Organic Chemistry – IUPAC Recommendations and Preferred Names 2013 (Blue Book) reads as follows:
P-14.4 NUMBERING
When several structural features appear in cyclic and acyclic compounds, low locants are assigned to them in the following decreasing order of seniority:
(…)
(e) saturation/unsaturation:
(i) low locants are given to hydro/dehydro prefixes (…) and ‘ene’ and ‘yne’ endings;
(ii) low locants are given first to multiple bonds as a set and then to double bonds (…);
(f) detachable alphabetized prefixes, all considered together in a series of increasing numerical order;
(…)
Note that Rule e takes precedence over Rule f.
For example, a preferred IUPAC name is 3-bromocyclohex-1-ene (not 1-bromocyclohex-2-ene): (This example is given in the Blue Book.)

P-14.3.5 Lowest set of locants
The lowest set of locants is defined as the set that, when compared term by term with
other locant sets, each cited in order of increasing value, has the lowest term at the first
point of difference; (…)
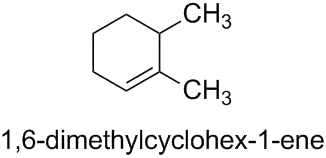
Therefore, the structure given in the question is named as 1,6-dimethylcyclohex-1-ene rather than 2,3-dimethylcyclohex-1-ene since the locant set ‘1,6’ is lower than ‘2,3’.