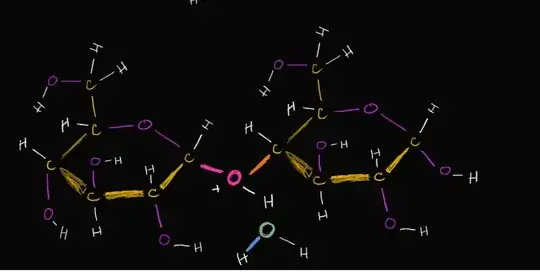
For example, oxygen is neutral when it has just 6 electrons. But the pink O in the molecule pictured has 3 bonds giving it 8 electrons, a full-octet. It's more electronegative than all the atoms it's bonded to also. It would make sense if an O with a full-octet had a -2 charge since it has an extra 2 electrons.
But because it "owns" only 1 electron from each bond, the O has a positive charge. This doesn't make sense to me: if we don't count the extra electrons in its charge, why do we count them towards its octet?