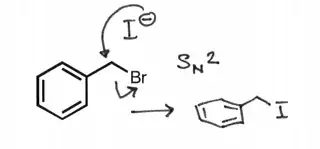
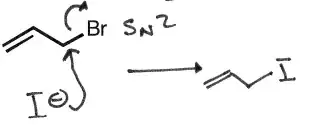My teacher stated that allylic carbocations are comparable in stability to tertiary carbocations. $\def\SN#1{\mathrm{S_N}#1}$
With this in mind I am confused why question 13 of this MIT practice test states that these two molecules are only expected to undergo $\SN 2$ reactions:
An $\SN 1$ reaction on the first molecule with the phenyl substituent would yield a primary carbocation, yes, but this primary carbocation is also benzylic, and can be stabilized through resonance.
An $\SN 1$ on the second molecule similarly yields a carbocation, but this is an allylic carbocation, and can easily be stabilized through resonance and delocalization of pi-electrons.
So, would $\SN 1$ be valid pathways of reactions for both of these molecules, or is $\SN 2$ simply the only pathway for these molecules to react?