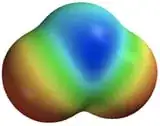
This is the electrostatic potential for ozone.
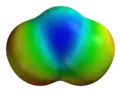
This is the electrostatic potential for sulfur dioxide.
They look almost exactly the same except one is all oxygen atoms and the other has sulfur in the center instead of oxygen.
Why does ozone have an MEP (molecular electrostatic potential) similar to that of SO2?