In benzene and 1,3-cyclohexadiene, resonance will occur, so both will have a partial pi character. Technically, all bonds in benzene have partial pi character, whereas in 1,3-cyclohexadiene, some have partial pi character and the rest are pure sigma. So considering this, 1,3-cyclohexadiene must have a greater bond length. But the answer in the book says benzene.
-
2Would be nice to point at the bonds you are looking at. Your text assume equal bond length which is true only in benzene (and is even the underlying answer to your question). – Alchimista May 18 '21 at 09:55
-
Sorry, I meant the C=C bonds. Have changed the question. – confused_Student May 19 '21 at 14:57
-
1In benzene, the double bonds are not localized. There are no real double bonds. Each C-C bond is of an order situated between $1$ and $2$. – Maurice May 19 '21 at 18:50
1 Answers
It would be interesting to know the sources cited in your question (both the book, as well as the primary literature) as reference because one complementary approach to the (assumed) spectroscopic determination is the analysis of the crystalline state. While there are multiple records about pure benzene, it seems there is no public model of the crystalline structure of pure 1,3-cyclohexadiene ; on the other hand, there are multiple records where the two motifs are present simultaneously.
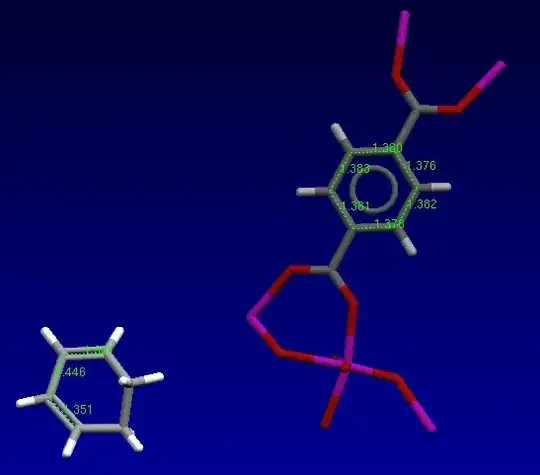
From those with little geometric distortion by organometallic bonding, a framework of 1,4-benzenedicarboxylates with incorporated (guest molecules) of 1,3-cyclohexadiene nevertheless appears instructive:
(CCDC entry IXODUV, based on Wang et al. in 2011InorgChem2028)
For the moderately distorted 1,3-cyclohexadiene, the intermolecular distances are $\pu{1.35 A}$, $\pu{1.45 A}$, and $\pu{1.33 A}$ along the sequence of double, single, double bond $\ce{C=C-C=C}$. In the p-terephthalate, the model describes all aromatic $\ce{C\bond{~-}C}$ bonds with $\pu{1.38 A}$. Despite the constraints of the structure model, it is an example for the difference of aromatic $\ce{C\bond{~-}C}$ bonds from conjugated double bonds.
In the context of your question, the paper is instructive because the framework of the host, the p-terephthalate, chemically stays the same and with only minor changes of the lattice constants if it is loaded with guest molecules of benzene, 1,3-cyclohexadiene, 1,4-cyclohexadiene, cyclohexene, or cyclohexane.
- 29,590
- 2
- 45
- 108
-
1I'll guess: The question comes from an exercise book which is designed to "teach" rules of thumbs and educated guessing concepts. As such the target of this was probably to count some resonance structures with charge separation and seeing that the others they are equal in benzene and that is much better and whatnot and then conclude that there is more delocalisation in benzene than there is in the other one and hence the double bond in benzene is less of a double bond and must be weaker and therefore longer. Anyway, I really like your very scientific answer to such a terrible exercise. – Martin - マーチン May 19 '21 at 22:27