The (cis)/(trans) nomenclature (or, more universally applicable, (E)/(Z) nomenclature) works fine for isolated and conjugated double bonds. In the case of 2,3-pentadiene, however, where double bonds literally are adjacent to each other, you have a cumulene. As a result, the mutual orientation of two terminal methyl groups is close to perpendicular.
To put things in perspective (literally), build a model with a model kit:*
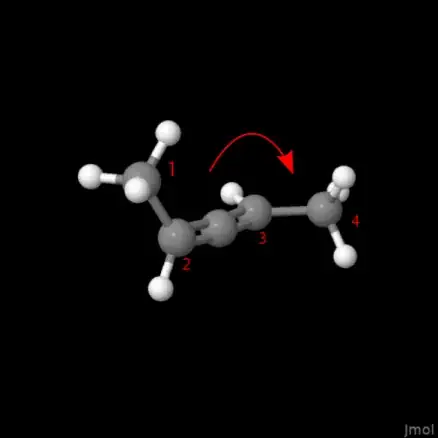
and apply the nomenclature for axial chirality instead. You assign the methyl groups a higher priority than the hydrogen atoms (CIP rules), then, while looking along atoms (2) and (3), the «rotation» along the sequence 1 -> 2 -> 3 -> 4 is clockwise, thus it is (2P)-penta-2,3-diene (where P (as in plus, to the right, clockwise), and M (as in minus) is used to describe the axial chirality and helical chirality.
The relevant rules in the Blue Book (see resources page) are P-92.1.2.1, P-92.1.2.1.2, P-92.1.2.2.1, and P-92.1.2.2.3. If you want to continue to use (R) and (S) to assign axial chirality, add a subscript to the label (here, they correspond inversely and hence (2P)-penta-2,3-diene is ($2S_\text{a}$)-penta-2,3-diene).
*In case you do not have access to a model kit, you may paste the following SMILES string (a structure description a [dedicated] computer program understands) CC=[C@@]=CC as input for molcal.org. Don't mind that the angle enclosed by the methyl groups differs from the one you get with a physical model kit (example), focus on the relative orientation.
Reference:
Favre, H. A., Powel, W. H. Nomenclature of Organic Chemistry. IUPAC Recommendations and Preferred Names 2013. Excerpt prepared by Moss G. P. at https://iupac.qmul.ac.uk/BlueBook/
