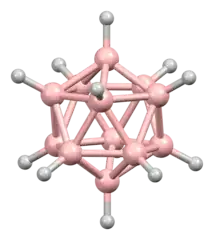
Boron makes not five but six bonds in compounds such as dodecaborate or boron carbide. Boron carbide is a semiconductor, so it has aspects of a metal and a non-metal. For actual metals such a elemental sodium, you have to explain twelve nearest neighbors with only 1 outer electron, so there is no way to describe it with covalent bonds. For boron carbide, you can get away with invoking 3 center 2 electron bonds, but it is not clear if there is much insight to be gained.
Roald Hoffmann wrote a detailed account of bonding in boron carbide here. Even if all you get out of it is that boron makes beautiful intricate structures, and 2 electron bonds do not do justice to all compounds known, it is worthwhile taking a look.

I don't think any of the bonding models make use of the inner electrons.