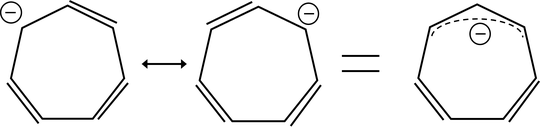
The $\mathrm{p}K_\mathrm{a}$ values of methylene protons in cycloheptatriene vs cyclopropene are found to be 60 and 36, respectively.
From what I understand - both conjugate bases are non-aromatic (not anti-aromatic) as a result of some sort of Jahn-Teller distortion. I can't pin down the nature of the distortions but they appear to be a pyramidal geometry for the cyclopropenyl anion and either $\mathrm{sp^{2}}$ hybridisation or unequal bond lengths for the 'tropylium' anion.
In either case, if both species and both conjugate bases are non-aromatic what is the cause of the massive $\mathrm{p}K_\mathrm{a}$ difference? Is it something to do with the $\mathrm{sp^{2.68}}$ hybridisation in the three membered ring?