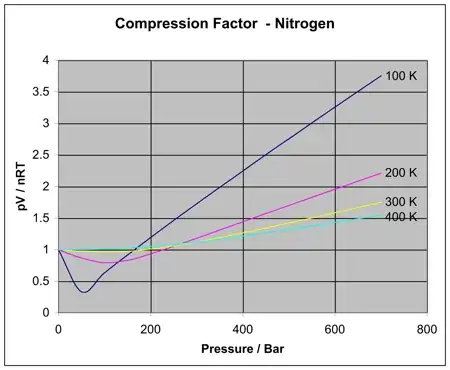
Since the pressure of a real gas is less than that of the ideal gas and its volume is more than that of ideal gas, I am assuming that the real gas is difficult to compress in comparison to an ideal gas.
Is my logic right? Also I'm looking for a clearer explanation!