I think cyclobut‐3‐ene‐1,2‐diide is non-aromatic since there are two sp3 carbons (carrying negative charge), but my source says it is aromatic. Why?
-
1https://www.masterorganicchemistry.com/2017/03/03/is-this-molecule-aromatic-some-practice-problems/ – Mäßige Aug 02 '22 at 07:22
-
1https://chemistry.stackexchange.com/a/18566/9961 – Mithoron Aug 02 '22 at 12:24
-
simplified answer-the carbons are not sp3 because lone pairs delocalized by resonance are not counted in the hybridization of the atom – Boson Aug 02 '22 at 17:29
1 Answers
Just because a carbon atom is negatively charged and has a lone pair, it need not be $sp^3$ hybridized. The lone pair could be on a $\pi$-bonding $p$ orbital with the atom hybridized $sp^2$. If you allow for this possibility, you find that the given ion can satisfy the $4n+2$ rule for aromaticity, which the text apparently is claiming.
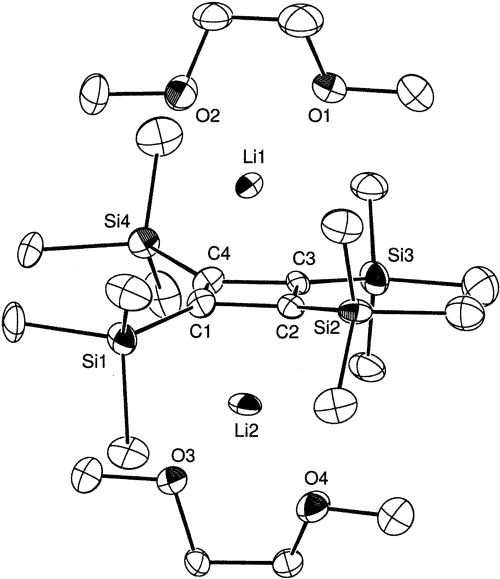
Whether the ion is actually a stable aromatic species is more complicated. Formation of a highly strained ring, the high charge density, and a relatively low amount of resonance stabilization for four-member aromatic rings all work against forming a strongly stabilized ring (the cyclopropenyl cation is also highly strained, but it has less charge density and greater resonance stabilization). Known cyclobutadiene-dianion compounds are stabilized by using substituents that allow some dispersion of the charge and closely associating the counterions above or below the ring. See for instance References 1 and [2].
Structure of a lithium cyclobutadienide complex, from Ref. [2]. Unlabeled atoms in the ring substituents and solvent molecules are carbon; hydrogen atoms are not shown.
References
Akira Sekiguchi, Tsukasa Matsuo & Hidetoshi Watanabe (2001). "Cyclobutadiene Dianion Dilithium: a New Aromatic Ring System**. Phosphorus, Sulfur, and Silicon and the Related Elements 168:1, 51-58, https://doi.org/10.1080/10426500108546530.
Akira Sekiguchi, Tsukasa Matsuo, and Hidetoshi Watanabe (2000). "Synthesis and Characterization of a Cyclobutadiene Dianion Dilithium Salt: Evidence for Aromaticity". J. Am. Chem. Soc. 122, 23, 5652–5653. https://doi.org/10.1021/ja0004175
- 56,895
- 4
- 89
- 175