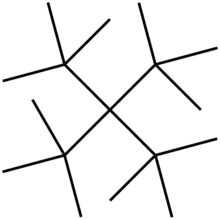
Does this molecule exist? I've been wondering about extreme steric hindrance and trying to find the limit of what can exist and what not. It appears to exist some basic data about it in PubChem, but does this really matter? Do all the molecules that are recorde in this site really exist? I haven't found anything more, and I would also like to know if there's any synthetic path to achieve it. Maybe it's impossible to know how to produce this molecule, but I'm sure that is possible to calculate the thermodinamic parameters or the relative stability.
Wikipedia also shows that it's an hypothetical compound and there are energetic studies in references: ...for tetra-TBM, a compound that has not yet been synthesized, the G3(MP2) results reported in this work should be reliable estimates...
So, does this mean that is possible to make it but it hasn't been made yet?
https://pubs.acs.org/doi/pdf/10.1021/ci0497657