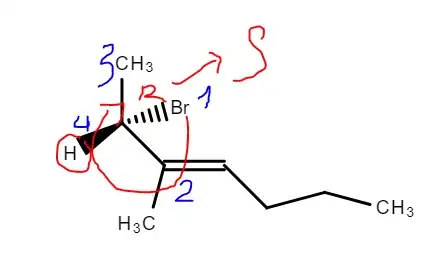
I'm trying to find the R/S configuration of this molecule right here:

At first I tried to find the rotation and then reverse it (since H is pointing outwards), I got an S configuration (drawn poorly here):
Then I tried to use the enantiomers method. I swapped the Br and the H then tried to find the configuration of the new molecule. And, I got an S configuration (of the resulting new molecule, which leads to the original having R configuration), which is not what I expected:
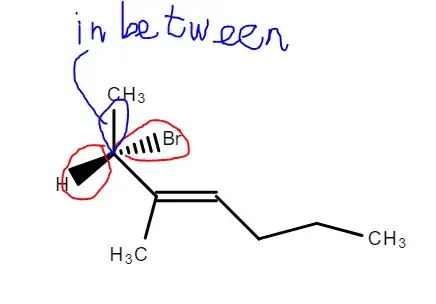
I don't know what I've done wrong here, the two attempts should lead to the same result (unless I've done something wrong). I tried looking up other R/S config examples using the enantiomers method (switching method) and what I've noticed is that the wedge and the dotted lines are right next to each other, like this for example:
While the molecule above has a straight (?) line in between:
So does that mean I can only switch groups when the wedge and the dotted lines are next to each other? Or was the first method wrong? Please kindly explain. Thank you.
Update 1 I've added a 3D visualization of the molecule. The big red ball is bromine, the gray medium-sized balls are carbon atoms, and the little white ones are hydrogens. I've marked the chiral center (green arrow pointing to it) for clarity. I've also rotated the molecule so that the bromine is pointing outwards.