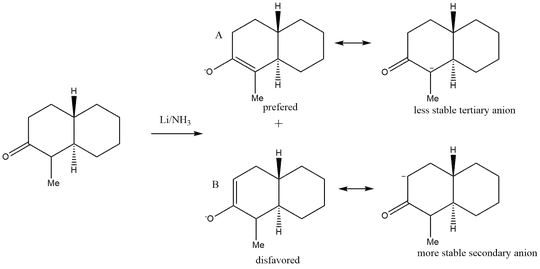
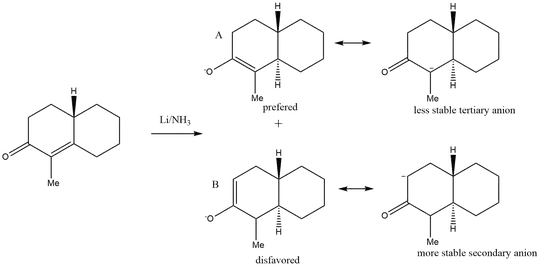
Unsymmetrical ketones can produce two different enolates, as shown in your reaction scheme. The enolate pictured in the top row of your drawing is referred to as the thermodynamic enolate, while the enolate shown in the bottom row of your drawing is the kinetic enolate.
More highly substituted double bonds are thermodynamically more stable than less substituted double bonds. Therefore, the thermodynamically favored enolate is the one that gives rise to the most highly substituted double bond in the enolate intermediate. The thermodynamic enolate will be formed when reaction conditions are chosen (see below) to allow equilibration of all enolate intermediates; product formation is then thermodynamically controlled.
The kinetically favored enolate is the fastest formed enolate, the one formed by abstracting the most acidic proton (proton acidity follows primary > secondary > tertiary). In your reaction, removal of the proton on the secondary carbon would be kinetically favored (more acidic) over removal of the proton on the tertiary carbon atom (less acidic).
Reaction conditions can be selected to favor kinetic (aprotic solvents, strong bases, low temperature) or thermodynamic (protic solvents, weaker bases, higher temperature) enolate formation, giving the chemist control over selecting which product will be formed.
If your reaction were run under conditions favoring kinetic enolate formation, then we would expect to observe product formed from following the bottom reaction line. If the reaction were run under conditions favoring thermodynamic enolate formation, then we would expect to obtain products from following the top line of your reaction scheme.
So if your professor said that pathway "A", the top line in your drawing, is favored, then the reaction is being run under conditions favoring formation of the thermodynamic enolate.