I'm reading Unit operations of Chemical Engineering, 5th edition by Warren McCabe.
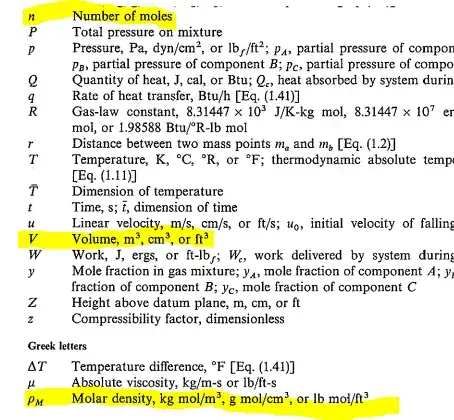
They defined the "molar density $\rho_{M}$" as $\ce{\rho_{M}=\frac{n}{V}}$. The parameters $n$ and $V$ are the number of moles and volume as usual (they are indicated in the summary table of all the symbols at the end of the chapter). To my understanding, $\rho_{M}$ indicates how many moles of a molecule are there in a volume unit and its unit should be something like $\ce{mol/m^3}$ or $\ce{mol/cm^3}$.
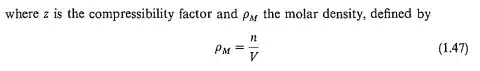 $\rho_{M}$" />
$\rho_{M}$" />
However, the unit of $\rho_{M}$ in that table is $\ce{kg\cdot mol/m^3}$. Why is there the presence of mass?