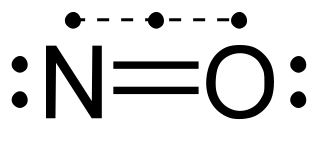Here is the most common way of writing the Lewis structure of NO, a molecule with an uneven number of valence electrons:

Source: http://www.chem.ucla.edu/~harding/IGOC/N/nitric_oxide01.png
From that image, you would say that there are two lone pairs on the oxygen atom (that is the typical binding pattern) and one lone pair and an unpaired electron on the nitrogen.
However, the Lewis structure does not reflect that the bond is a bit shorter than the N=O double bond found in other molecules and ions (and a bit longer than the triple bond).
If you take a look at the MO-diagram, you will see three pairs of electrons designated as bonding, and a single electron as antibonding (if we call the electrons in the 2s orbitals non-bonding for now).

Source: https://chemistry.stackexchange.com/a/15317
There is no good way to draw this as a Lewis structure. It seems like Wikipedia tried something without explaining it:
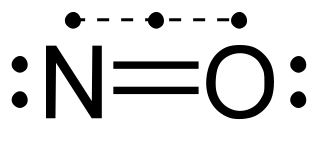
Source: https://commons.wikimedia.org/wiki/File:Nitric_oxide.svg
This depiction, however, is confusing. There should never be three electrons in an orbital.
You could invent a new way of drawing Lewis structures, showing a triple bond plus an electron that is designated as antibonding. It would look like this (but it should be emphasized that this is a non-standard depiction):

However, if you take this as the Lewis structure, you would count two lone pairs. Hopefully, this discussion shows you that lone pairs are not well-defined if you can't draw a Lewis structure that captures the molecule well.