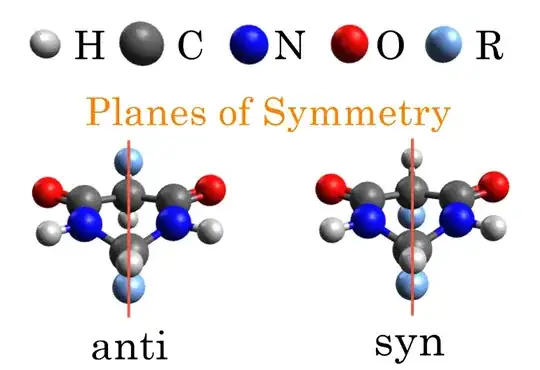
If we add substituents R to this structure, we can have two diastereoisomers with the two R groups on the same side (syn) of the flat ring or on opposite (anti) sides. Although the plane of the paper is no longer a plane of symmetry, neither isomer is chiral as the other plane bisects the substituents and is still a plane of symmetry.
Asked
Active
Viewed 128 times
-2
-
4The plane of symmetry contains both R's and both H's, so they don't move when reflected. – orthocresol Jun 04 '23 at 21:13
-
2Exactly like it is drawn in the picture... – Mithoron Jun 04 '23 at 23:22
2 Answers
2
The two $\ce{CHR}$ groups at opposite corners of the ring are co-planar, and both isomers are symmetric about this plane.
Yes, seeing a molecule in three dimensions when you have a paper drawing is difficult. In my day at school the recommendation was to use a kit to build a molecular model, which you could rotate to get different views. Today we can use computer images.
Mithoron
- 4,546
- 14
- 40
- 61
Oscar Lanzi
- 56,895
- 4
- 89
- 175
-
So, what is wrong this time? (Other than getting burned by Autocorrect, which forced an early-morning edit?) – Oscar Lanzi Jun 05 '23 at 09:35
2
Both molecules have a plane of symmetry. The $\ce{R}$ groups lie on the plane of symmetry. Try visualizing it this way:
Unless the $\ce{R}$ groups are chiral, both the molecules can exist in an achiral conformation.
Also, likely, the ring is not planer because $\pu{120 ^\circ}$ is not the natural angle for the $\ce{N}$ atoms in (somewhat) $\mathrm{sp^3}$ hybridization.
ananta
- 2,289
- 3
- 22
-
3
-
@orthocresol 'very much' being the keywords, the six-membered ring is strained in the planer configuration. Based on geometrical optimization, non-planarity adds stability. – ananta Jun 05 '23 at 13:24