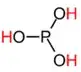
As an oxyacid, Phosphorous acid intuitively should have the structure
since the hydrogens should be on the oxygens.
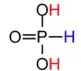
However, the major form of this acid is actually
with the hydrogen attached to the phosphorous.
My question is, what fundamentally causes the second form to be more stable than the first?