I was practicing writing nomenclatures for several organic compounds with the help of Blue Book Essentials and I have some queries regarding the following structure:
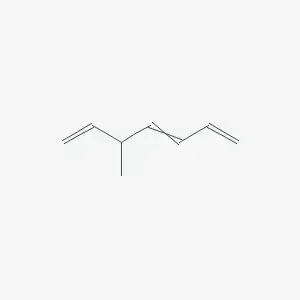
According to the blue book, the numbering of parent chain is as follows:
- Lowest locants for heteroatoms
- Lowest locant(s) for indicated hydrogen
- Lowest locant(s) for principal characteristic group(s)
- Lowest locants for ‘ene’, ‘yne’, and hydro prefixes
- Lowest locants as a set for all substituents cited by prefixes
- Lowest locants for substituents in the order of citation.
According to this order, the carbon bearing the methyl group should get the lesser number, hence '3'. So the name should be 3-methylhepta-1,4,6-triene?
Please clarify this for me. Thanks!