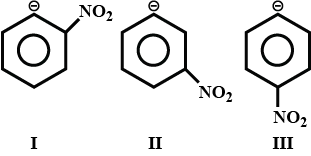
First off, the mesomeric (M) effect applies only when pi-electron conjugation is operating, and here it is not. When you deprotonate benzene, the excess electron pair left behind is in the plane of the ring where the hydrogen was; thus not conjugated with the pi system.
It is true that the inductive effect is strongest with the negative charge at the ortho position, but you have to reckon with steric effects. A nonbonding electron pair is more bulky than a bonding pair, especially when the bond is with hydrogen; so deprotonating the nitrobenzene at the ortho position invites interference and repulsion between the electron pair left on the carbon and those on the adjacent nitro group. You need to go to the meta position to keep the two sets of nonbonding electrons far enough apart for the stability you expect on charge-distribution grounds. This does not mean the more favorable steric effect with the meta isomer overcomes the stronger inductive effect with the ortho isomer, but you have to assume that might happen.