I basically agree with Ron's answer, but had to draw all of the possible structures to confirm it. The complication is that carbon-2 is non-stereogenic but it may be chirotopic depending on the configuration of the neighboring atoms. The IUPAC Gold Book calls this a pseudo-asymmetric carbon atom. Carbon-2 is not a stereocenter, because it does not have four different "things" attached to it. However, its configuration is important relative to carbons-1 and -3. Below are all of the possible combinations.
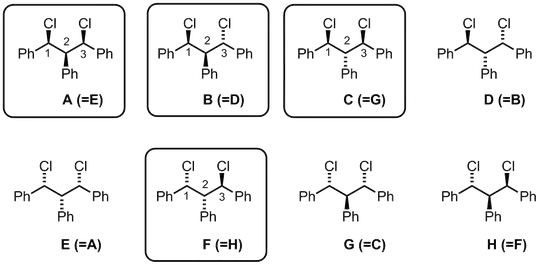
Out of the eight possibilities, we see there are only four unique compounds (names generated in ChemDraw):
A: (1R,2s,3S)-1,3-dichloro-1,2,3-triphenylpropane
B: (1S,3S)-1,3-dichloro-1,2,3-triphenylpropane
C: (1R,2r,3S)-1,3-dichloro-1,2,3-triphenylpropane
F: (1R,3R)-1,3-dichloro-1,2,3-triphenylpropane
The small r and s are essentially used to indicate the stereochemistry at carbon-2 relative to carbons-1 and -3. Note that in compounds B and F, the stereochemistry at carbon-2 is inconsequential as in both cases the phenyl group is syn to one chloride and anti to another chloride. I haven't been able to find an adequate explanation of assigning the r/s designations, but I believe that the R configured substituent receives higher priority than the S configured substituent, and Cahn–Ingold–Prelog system is followed from there.


