The carbon is $\mathrm{sp^2}$ hybridised and is therefore planar and should also, theoretically be $120^\circ$. However, VSEPR theory suggests that the π bond would "need more space" due to greater electron repulsion. As a consequence the $\ce{H-C-H}$ bond angle would be smaller. However, since the π bond is out of the plane of the molecule does this actually happen?
2 Answers
The H-C-H bond angle in ethene is ca. 117 degrees and the H-C-C angle is ca. 121.5 degrees. There are two reasons that combine to explain this angular deformation in ethene.
First, from these bond angles and Coulson's Theorem (ref_1, ref_2) we can determine that the C-H sigma bonds are $\ce{sp^{2.2}}$ hybridized and the C-C sigma bond is $\ce{sp^{1.7}}$ hybridized.
From these hybridization indices (the index is the exponent "n" in the $\ce{sp^{n}}$ expression) we see that the C-C sigma bond has higher s-character content (1 part s to 1.7 parts p - 37% s) than the C-H bonds (1 part s to 2.2 parts p - 31% s). Since there is more s character in the C-C bond, it is lower in energy and the carbon sigma electrons will tend to flow towards this lower energy C-C bond. Consequently, the C-C sigma bond will contain more electron density than the C-H bonds. Therefore, the electron repulsion between the C-C sigma bond and C-H sigma bonds will be greater than the electron repulsion between the two C-H bonds. Hence the H-C-C bond angle will open up slightly from the $\ce{sp^{2}}$ ideal of 120 degrees and the H-C-H angle will close down slightly in order to minimize the bond-bond electrostatic repulsions.
Second, steric factors (which are also really just another way of describing electron-electron repulsion) may also come into play. To whatever extent the cis H-C-C-H hydrogen-hydrogen repulsion is more destabilizing than the geminal H-C-H hydrogen-hydrogen repulsion, it will also serve to increase the C-C-H bond angle and shrink the H-C-H bond angle.
-
4The first statement is not entirely true. Since the pi-bond influences the bond distance it influences the overlap of the sigma-bond forming orbitals, hence also influencing the sigma system, even if the orbitals are orthogonal. An observational explanation is given with Bent's rule. – Martin - マーチン Jan 01 '15 at 16:35
-
@Martin Excellent point Martin, I'll edit my answer. – ron Jan 01 '15 at 16:38
-
Do pi electrons repel the CH sigma electrons? – Dissenter Jan 01 '15 at 18:11
-
@Dissenter Yes they do. – ron Jan 01 '15 at 18:12
-
@Ron okay, thanks for the clarification. Does that make this point moot? " Yet it still causes the bond angles of substituents in a perpendicular plane to change? Is that right? Is it purely Coulombic interactions that causes this - it seems odd given that it's perpendicular to the plane." – Dissenter Jan 01 '15 at 18:13
-
3@Dissenter The electron density in the "top" of the pi cloud will tend to repel the C-H bonding electrons and push the down; but the "bottom" of the pi cloud will push them up. By symmetry these effects must cancel and the result is no net up-down C-H deformation due to the pi cloud. Also due to symmetry the pi cloud cannot push the C-H bonds out or in. – ron Jan 01 '15 at 18:18
-
@ron Sir "extent the cis H-C-C-H hydrogen-hydrogen repulsion is more destabilizing than the geminal H-C-H hydrogen-hydrogen repulsion, " can you give reason as to why its more destablizing ? And can from reason given here itself we can say HCH bond angle in ethane too will be slightly less than 109.5 ° ? – Orion_Pax Mar 29 '22 at 10:10
-
Also Sir pi cloud can push them out/in as in because the force on the c-h bonds are cancelling in perpendicular directions not along their directions i think , so it maybe a good reason to cause bond angle to decrease – Orion_Pax Mar 29 '22 at 10:33
In terms of the strongly simplifying VSEPR model, you could arrive at the same conclusion by noting that a carbon atom is larger than a hydrogen atom, so the $\ce{CH2}$ group will request more space than each hydrogen. You can compare this to ethane $\ce{H3C-CH3}$, which has $\angle(\ce{H-C-C}) = 111.17^\circ$, slightly deviating from the ideal tetrahedral angle of $109.5^\circ$ but no π system to explain the deviation. If I did the maths correctly, that means that $\angle(\ce{H-C-H}) = 107.72^\circ$. The deviation in ethane is actually larger than that in ethene, suggesting that steric factors predominate.
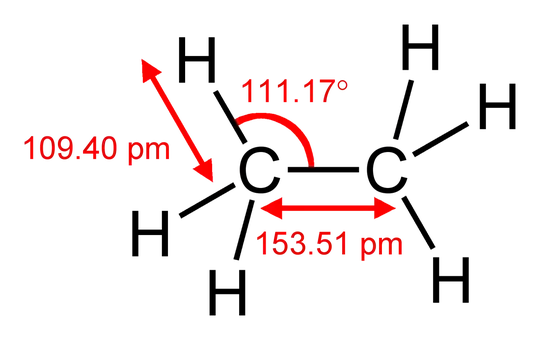
Figure 1: Ethane’s structure including bond lengths and angles. Taken from Wikipedia, where a full list of authors is available.
- 67,989
- 12
- 201
- 386
-
Although steric factors is a reason , can repulsion between different carbon hydrogens sigma electrons too can be said for a decrease in bond angle HCH Sir ? – Orion_Pax Mar 29 '22 at 10:13
-
Also Sir @Jan i think deviation here is a bit less(109.5-107.72) than ethene (120-117) – Orion_Pax Mar 29 '22 at 10:15