The Lewis structure of nitric acid is:
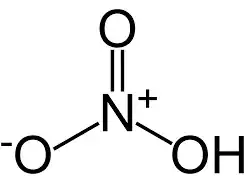
So, since Pauling's rule is $\mathrm{p}K_\mathrm{a}=8-5p$, where $p$ is the number of oxo groups, how is the rule applied in this instance? $p=1.5$? $p=2$?
The Lewis structure of nitric acid is:

So, since Pauling's rule is $\mathrm{p}K_\mathrm{a}=8-5p$, where $p$ is the number of oxo groups, how is the rule applied in this instance? $p=1.5$? $p=2$?