The book's explanation about a noble gas configuration is somewhat accurate, but fairly incomplete.
The elements on the right and on the left of the periodic table (the alkali (earth) metals, the halogens, the chalcogens (the group that starts with Oxygen) and the pnictogens (Nitrogen group)) have electron configurations that make it somewhat easier to lose/gain electrons to "look" like noble gasses (this is why alkali metals and halogens are so reactive: the destabilization from the charge is very minimal compared to the stability of the electron configuration).
However, as you have observed, the book goes to some effort to avoid talking about transition metals. There is a reason for that.
Noble gas configurations are a subset of the stable configurations of electrons. In reality, what's actually being aimed for is an element with no incomplete electron shells.
What is an electron shell? The actual quantum mechanical definition may be a bit more complicated than you need, but for your purposes, it suffices to say that it's how electrons will tend to be distributed around a nucleus. Note that this is extremely distinct from saying that the electron "orbits" the nucleus.
If you look at a periodic table, there are four general regions to look at:
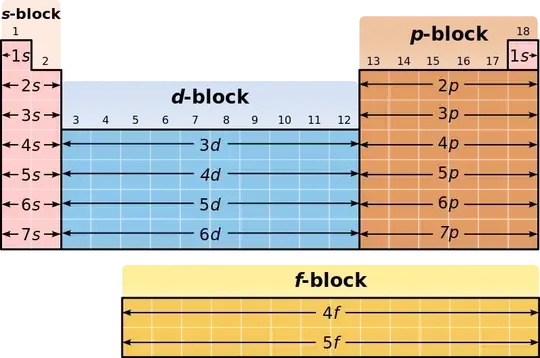
The labels "s", "p", "d", and "f" all refer to the various electron orbitals, and the blocks all indicate what the outermost (or "valence") shell is.
So while elements in the s and p blocks can lose a small number of electrons get complete shells (which will resemble the noble gases), transition metals are a bit more complicated. And by a bit more complicated, I mean that there are very rough rules in place that have almost more exceptions than they have actual examples of following the rule.
But in very general terms, a transition metal will lose electrons. If they are in the first half of the transition metals in their row, they can lose a number of electrons less than or equal to the number of d electrons plus the number of s electrons. This maximum number of electrons lost will make them resemble the noble gasses in configuration, but it's only appreciably stable when the transition metal is in the first half of transition metals in its group ($\ce{Mn^{7+}}$ is well documented in ionic compounds, but $\ce{Fe^{8+}}$ doesn't exist a lot because it has too many electrons to try to lose to comfortably fall into a noble gas configuration). But transition metals can lose electrons up to this point. The issue is, most transition metals can adopt multiple charge configurations for various reasons, so generally, when writing an ionic compound with a transition metal in words, you need to specify the charge of the transition metal (actually, its oxidation state, but in an ionic compound that's mostly splitting hairs). So you can have "Iron (II) Oxide", which is a black powder and "Iron (III) Oxide" which is rust. These compounds will have different chemistries because of the charge on the transition metal.
Suffice to say, your textbook is avoiding transition metals because transition metals are weird.
