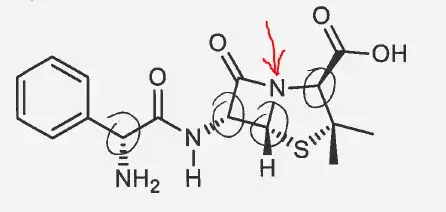
Shouldn't the N pointed to by the red arrow also be a stereocenter? I am asking this because I think it's lone pair cannot take part in nitrogen inversion.
Asked
Active
Viewed 2,658 times
11
-
1This is a very good question. I'm not an expert at N stereochemistry but have you considered the iminol form of the amide/iminol tautomer? It would have a double-bond to the N and would not be chiral. I know the amide form predominates but if there is any contribution from the iminol it would "destroy" the chirality wouldn't it? – Curt F. Jul 28 '15 at 05:43
-
Why do you think that nitrogen inversion cannot take place there? – pH13 - Yet another Philipp Jul 28 '15 at 06:08
-
1Probably because N is part of a ring but I am not sure if any strain is involved? – fidgetyphi Jul 28 '15 at 06:25
-
4The two connected rings and the attached sterically demanding groups will certainly hinder inversion – Martin - マーチン Jul 28 '15 at 07:10
-
2Amide groups are flat! There's no point in talking about inversion. – Mithoron Jul 28 '15 at 10:27
1 Answers
2
After reading the inputs from @Curt F., @PH13, @Martin and @Mithoron, I infer that N won't be involved in nitrogen inversion due to hindrance caused by connected rings and attached sterically demanding groups. Hence, 'N' pointed to by the red arrow will be considered as a stereocenter in ampicillin.
Mithoron
- 4,546
- 14
- 40
- 61
fidgetyphi
- 453
- 6
- 18
-
You didn't get my comment - it's flat so there can't be stereocenter! – Mithoron Jul 28 '15 at 11:13
-
@Mithoron Correct me if I am wrong, you said that amides are flat and therefore nitrogen inversion won't be possible, right? Now, the absence of nitrogen inversion facilitates in N being a stereocenter given that all the substituents attached to it are different as well (which is also satisfied in this particular case). – fidgetyphi Jul 28 '15 at 11:25
-
2All substituents of N atom are in one plane - thats why speaking of prescence of inversion or lack of inversion doesn't make sense. – Mithoron Jul 28 '15 at 13:06
-
@Mithoron So, are you implying that a 3-D geometry is a requirement for an atom to be a stereocenter? Because I don't see it in any of the definintion of stereocenter I found on the internet. One of the legit sources being: http://www.chem.ucla.edu/harding/tutorials/stereochem/id_stereocenter.pdf – fidgetyphi Jul 28 '15 at 13:18
-
2@Mithoron I think you should write up an answer. Also everyone please avoid extended discussions in comments. Use the chatroom instead. – bon Jul 28 '15 at 13:20
-
1@theprogrammer see discussion starting here http://chat.stackexchange.com/transcript/message/23058334#23058334 – Mithoron Jul 28 '15 at 14:06
-
I edited your answer - as you could see in discussion I linked the amide group won't be flat. – Mithoron Jul 29 '15 at 21:45
-