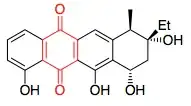
I'm trying to work out which are the aromatic ring systems in the drug aklavinone:
I am trying to follow Hückel's rules that the compound must be cyclic, be planar, possess a p-orbital on every atom in the ring, and have a π-system with $4n+2$ electrons.
I see that the benzene rings are obviously aromatic. But does the quinone ring (in red) also count as part of the 'aromatic ring system'?