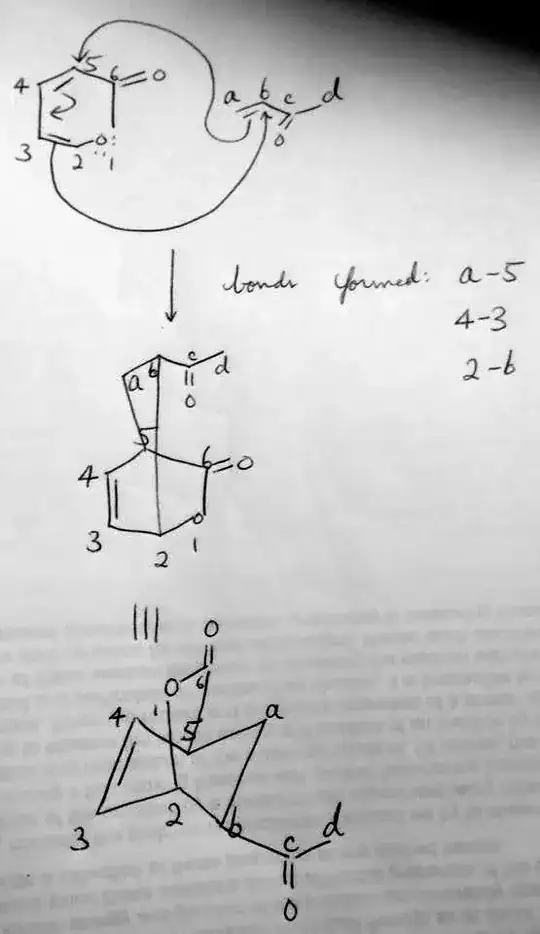
1) The regiochemistry of the product can be predicted by drawing resonance structures of the diene and dienophile, allowing us to assign partial charges to the reactive centers. Doing that shows us that 2 and a are partially positive while 5 and b are partially negative. We'd then predict that 5 attacks a and b attacks 2. This answer has a figure showing prediction of regiochemistry this way.
2) We would predict that the product is the endo- product, which would have the bridging ester on the opposite side of the cyclohexene relative to the methyl ketone. This has to do with the endo-rule, which is classically explained by secondary orbital overlap between the the lobe of the HOMO on carbon 4 interacting with the lobe of the LUMO on carbon c. Keep in mind that this trend does exist, but mixtures of endo and exo are common.