Question: Are these compounds enantiomers?
Attempt: The compounds given are mirror images. But if I rotated the three bonds in the third carbon (configuration will not change), I will get a meso compound (plane of symmetry) :
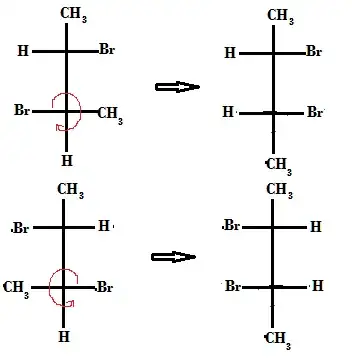
So are these compounds enantiomers or meso compounds?
