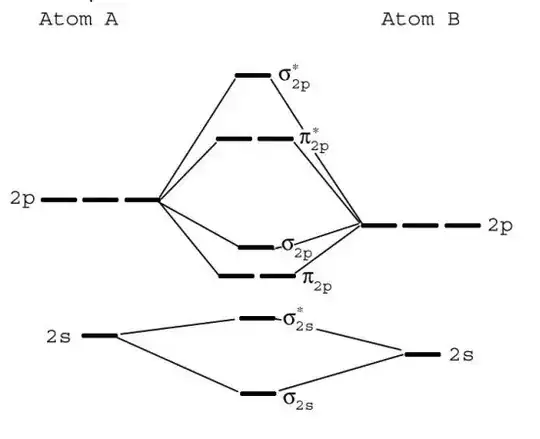
So in the picture above, see that there is an Atom A and Atom B and they combine. Notice that Atom A is higher in energy than Atom B (as indicated by how the 2s and 2p lines are drawn higher). Suppose I say that this is the diagram for NO. How would I tell which atom and which is O? What determines which atom has higher energy in the orbitals?
Thanks.