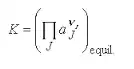In this equation that relates Standard free energy and the equilibrium constant:
$\Delta Gº=-RT \ln K_{eq}$
My textbook (and my teacher) say both $K_p$ (constant related to pressure) and $K_c$ (constant related to concentration) can be used in the place of $K_{eq}$.
When using the equation that relates $K_p$ with $K_c$
$K_p=K_c(RT)^{\Delta n}$
We would get two different $\Delta Gº$ values, which shouldn't be possible.
My question is, why can we use both constants if their values are different?