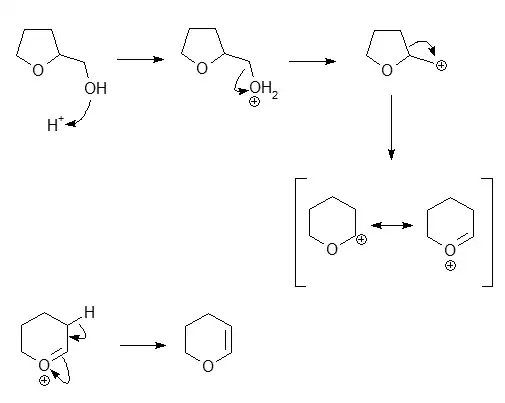
I think the ethereal oxygen in THFA gets protonated. Due to greater +I effect, the electron density on it is higher, hence it is more likely to behave as a Lewis base. Consequently, the product formed is pent-2-en-1,5-diol:
Apparently, this is wrong. What exactly happens here and why my answer is wrong?