My professor said while explaining the acidic nature of phenol that meta-nitrophenol is less acidic than para-nitrophenol, and gave the reason that resonance doesn't play on meta, but I didn't get it.
-
10Hi Rishabh, welcome to Chemistry Stack Exchange! Have you tried drawing the resonance structures of the meta-nitrophenol and para-nitrophenol ions? Hint: you should be able to draw 2 for the para one but none for the meta one. – Nanoputian Apr 24 '16 at 05:48
2 Answers
The acidity of nitrophenols (or any acid for that matter) is determined by the stability of the conjugate base. In the case of m-nitrophenol and p-nitrophenol, the relative stability can be determined by looking at the resonance structures.
You can see that p-nitrophenol has an additional resonance structure where the negative charge is delocalised onto the oxygens of the nitro group, which are highly electronegative and therefore stabilise the charge effectively. This stabilisation is not possible in the case of m-nitrophenol because there is no opportunity via resonance for delocalisation onto the nitro group.
- 56,895
- 4
- 89
- 175
- 15,369
- 13
- 62
- 91
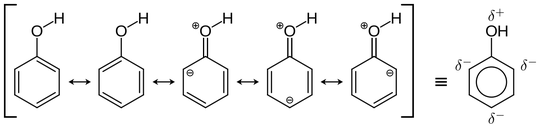
Refer to the structures the negative charge never gets over META position and hense resonance does not affect the electron density at META.
and hense nitro on META will not be able to pull electron from ring via (-M) and hense there would be negligible efffect on polarity of O-H bond.
- 43
- 1
- 7
-
7The oxygen in phenol definitely doesn't carry a partial positive charge. It is still very electronegative and bears a negative charge. – bon May 12 '16 at 18:02
